Despite FDA Proposals, 2020 Starts With Hundreds of Drug Price Hikes
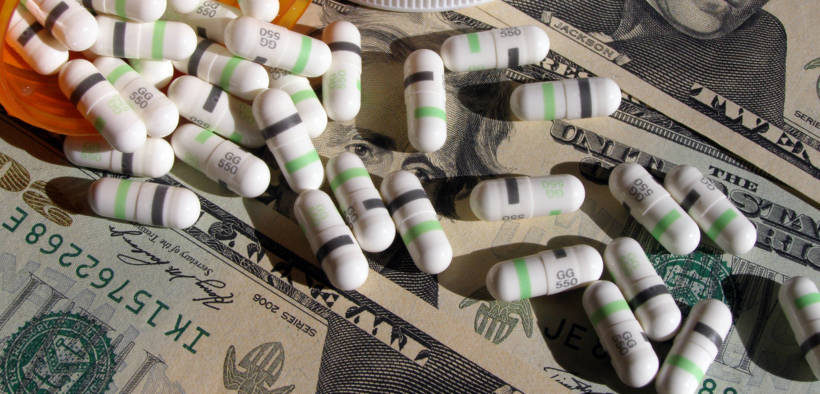
The FDA is attempting to tackle the high costs of prescription drugs with two new proposals, but the start of 2020 suggests the FDA’s efforts may backfire.
Although a new year is usually cause for celebration, patients who rely on prescription drugs have 407 reasons for dismay. As of Jan. 3, the research team at GoodRx indicated U.S. pharmaceutical companies hiked the prices of 407 drugs. The report came on the heels of a Dec. 18 Food and Drug Administration (FDA) press release promoting new rule changes which could allow the importation of drugs from Canada, but the process is not quick nor is the outcome assured.
What is the FDA Doing?
Simply put, the FDA issued a notice of proposed rulemaking (NPRM), the first step in codifying a policy change. An NPRM essentially is the agency’s way of letting the public know it is considering a change to its regulatory statutes. Furthermore, by law, it is an open invitation for comments on the idea. Anyone – from experts to medical patients to caretakers – may comment through the organization’s online system at regulations.gov or by mailing written concerns to Division of Dockets Management Room 1061, 5630 Fishers Lane, Rockville, MD.
The FDA proposed the NPRM for the importation of Canadian drugs alongside a proposed new draft guidance designed to give manufacturers guidelines on how to submit their products for FDA approval. This policy framework would instruct businesses on the steps they should take in order to market their products in the U.S. and, hypothetically, streamline the approval process.
Public comment for both the NPRM and draft guidance are open until Mar. 7 and Feb. 21, respectively. Afterward, the agency would need to approve the changes and they would take effect at a time determined by the FDA’s discretion.
Why are the Changes not a Silver Bullet?
“Today’s announcement outlines two pathways for the safe importation of certain prescription drugs to help provide safe, effective, more affordable drugs to American patients,” said Alex Azar, secretary for the Department of Health and Human Services in the Dec. 18. FDA press release. “These are historic actions by HHS and the FDA, and they represent the bold nature of President Trump’s agenda for lowering drug costs. The President has recognized the opportunity to lower costs for American patients through safe importation, and we at HHS and FDA are delivering on that possibility through a safe, commonsense approach.”
Azar’s comments portray the measure as a silver bullet to conquer rising medical costs and while the measure would indeed open another option for U.S. customers, it excludes insulins and biologics, one of the latest forms of medicine. Furthermore, the plan could actually backfire by constraining the supply in Canada. Additionally, there is no mechanism included in the proposal to prohibit a Canadian or U.S.-based importer from simply price-matching their U.S. counterparts rather than undercutting them.
The FDA’s press release is peppered with positive language, but completely devoid of statistics to support the measure. The idea of changing the agency’s rules is not new, however — a 2010 study calculated the Canadian drug supply would run out in less than a year if only 10 percent of U.S. orders were filled by imports, said Allison Martell for Reuters.
Furthermore, Canadian pharmaceutical regulations may make all of the FDA’s talk a moot point anyway as it often disallows businesses from fulfilling foreign orders. Governments of both nations have been lax about enforcing the policies for small shipments, however the Canadian authorities could simply begin giving them more scrutiny by enforcing the law to the letter.
Who Supports the Plan?
Not Canada. The nation is strongly opposed to the idea, primarily because its supply of drugs may lead to supply shortages. The plan could actually backfire and cause price increases. Although the former reason is a valid concern, the latter argument is a logical conclusion to the law of supply and demand, it may not hold as much weight due to recently-enacted price controls.
As prices climbed in the U.S. for hundreds of drugs, its northern neighbor is preparing for decreases. A law passed in August 2019 that will lower the cost of prescription goods in Canada will go into effect on July 1.
“Canada’s approach to drug pricing is unusual. Rather than bargaining prices down, the PMPRB declares that some prices are an illegal abuse of patent rights,” Martell wrote for Reuters. Critically, the Canadian Patented Medicine Prices Review Board will remove the U.S. and Switzerland, two of the most expensive nations for pharmaceuticals, from its price comparison list.
Pharmaceutical prices are the one point of near bipartisan agreement in Congress, but so far, bills have been stalled in both the House of Representatives and Senate.